Nov.07.2022
10100
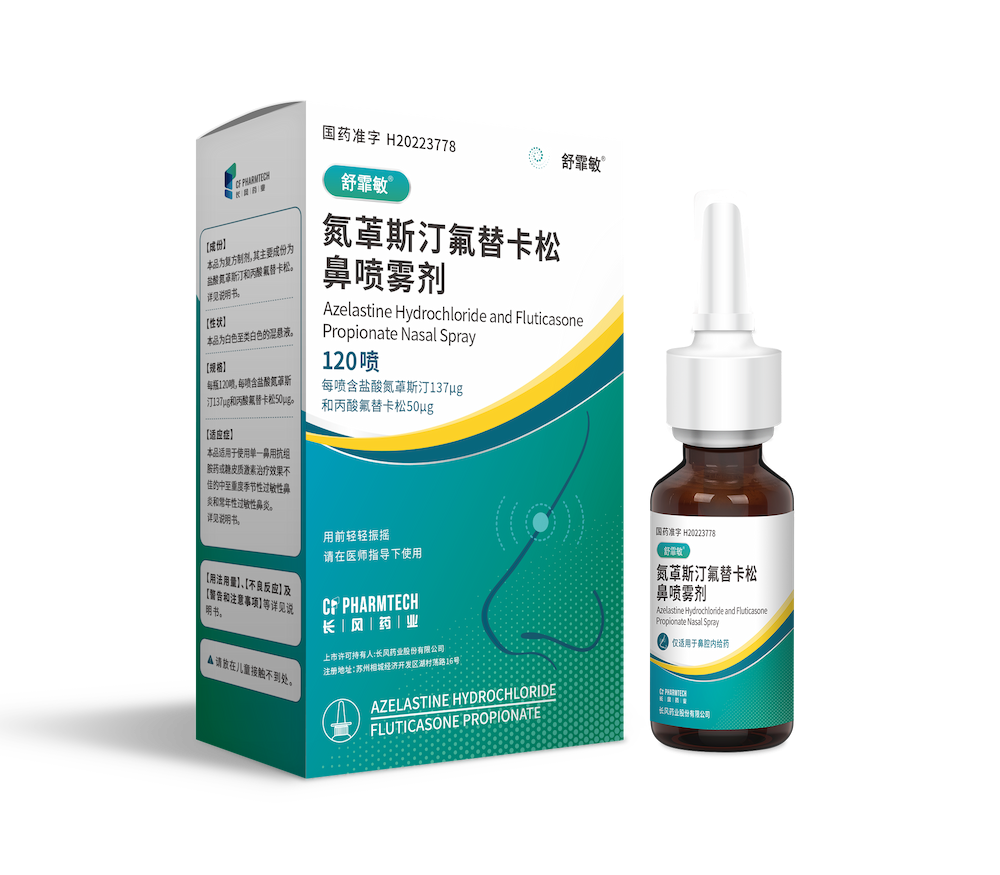
Today, CF PharmTech, Inc. (hereinafter referred to as: "CF PharmTech") independently developed the heavy product ShuFeiMin® (National drug approval number: H20223778, general name:Azelastine Fluticasone Nasal Spray, the specification is 120 sprays per bottle, each spray containing azelastine hydrochloride 137μg and fluticasone propionate 50μg) was the first approved in China.
This is the first domestic registration approval for a new treatment product in the field of allergic rhinitis in the world. The new product is a combination of nasal glucocorticoid and nasal antihistamine, which improves patient compliance and is easy to carry. ShuFeiMin® The market has ended the history of no hormone-antihistamine compound nasal spray in the domestic field of allergic rhinitis treatment, and has brought good news to the majority of patients suffering from the suffering of allergic rhinitis.
➣ Specific content
Azepine fluticasone nasal spray is used to treat adults and adolescents with moderate to severe seasonal or perennial allergic rhinitis. Intranasal administration provides both nasal hormones and antihistamines, the first-line recommended treatment for allergic rhinitis, at one time.
CF PharmTech in the spirit of excellence, fill the gap in the field of allergic rhinitis treatment, the benefit of the majority of patients, after ten years of efforts, through scientific research, and in the country to carry out Sufimin ® Phase III clinical trial, with solid research data support Shu Feimin ® Approved for listing in China.
➣ Epidemiology of allergic rhinitis
In recent years, with the deterioration of the global environment, air pollution and haze continue to worsen, the human body's autoimmune function is affected, and the incidence of allergic rhinitis is increasing year by year. According to the official statistics of WAO (World Allergy Organization), 10%-30% of adults and 40% of children suffer from allergic rhinitis worldwide [1]. According to epidemiological research in China, the number of patients with allergic rhinitis in China increased by about 100 million in the past six years, and the prevalence rate of allergic rhinitis was as high as 17.6%[2]. According to the statistical data of the seventh National population census, the population affected by allergic rhinitis in China has reached nearly 248.5 million, of which there are about 132 million patients with moderate to severe persistent allergic rhinitis [3], and the patient population is huge.
➣ Drug use in patients with allergic rhinitis
According to IQVIA data, the overall volume of China's anti-allergy chemical treatment market in 2019 increased by 13.6% compared with 2018, and the proportion of nasal hormones + nasal antihistamines accounted for about 25%. In 2021, China's main nasal hormones + nasal antihistamines market increased by 31% compared with 2019, with rapid growth. From the epidemiological data and clinical use, most patients with moderate-severe persistent allergic rhinitis have not received normative continuous treatment, and there is still a large number of clinical treatment needs that have not been released.
➣ Research progress in the treatment of allergic rhinitis
Chinese Guidelines for the Diagnosis and Treatment of Allergic rhinitis (Revised Edition 2022) pointed out that the treatment of allergic rhinitis should follow the treatment principle of "prevention and treatment, four in one", namely: environmental control, drug therapy, immunotherapy, health education. Drug treatment is an important part of this process. Nasal hormones and nasal antihistamines have the advantages of high local concentration and rapid onset, and lower risk of systemic side effects compared with oral treatment. After years of clinical use, they have become the first-line drugs recommended by domestic and foreign guidelines and the most common treatment drugs for allergic rhinitis [4].
。At present, nasal spray is the main therapeutic method for the clinical treatment of allergic rhinitis. As commonly used nasal antihistamines and nasal glucocorticoids, the combination of azepine hydrochloride and fluticasone propionate has outstanding therapeutic advantages. ARIA guidelines also state that combination therapy is necessary when symptoms of allergic rhinitis are not well controlled [5]. However, the use of two nasal sprays at a time requires a certain interval [6], and the phenomenon of drug dripping may occur when the two nasal sprays are used at the same time [7], which may cause the decline of patients' medication compliance, thus reducing the therapeutic effect of the drug. A large amount of evidence-based medical evidence [8-11] shows that azepine fluticasone nasal spray can more effectively relieve symptoms, improve the overall efficacy and have a good safety profile. Feimin Shu ® (Azepine fluticasone nasal spray) as the first new compound preparation in China, one spray can provide two first-line drugs, improve the convenience of use, and have the advantages of faster onset, stronger efficacy and better compliance, which can meet the treatment needs of patients with moderate to severe allergic rhinitis, and is a new breakthrough in the treatment of allergic rhinitis. It provides clinicians with more treatment options, while improving the effectiveness of treatment for patients with allergic rhinitis and the quality of life of patients.

Company profile
Since its establishment in 2008, CF PharmTech has been committed to the development of inhalation preparation technology platform and complex inhalation products, focusing on the integrated treatment of respiratory diseases. The company's research product line covers the treatment of major diseases of the respiratory system, involving allergic rhinitis, asthma, chronic obstructive pulmonary disease and many other therapeutic fields with high clinical demand. At the same time, the company has a research and development and production platform for nasal sprays, quantitative inhalation aerosols, dry powder inhalers, atomized inhalers, soft aerosols and other important inhalation preparations. It is a drug research and development company in the field of respiratory diseases. Production and sales of deeply focused and global layout of the enterprise.
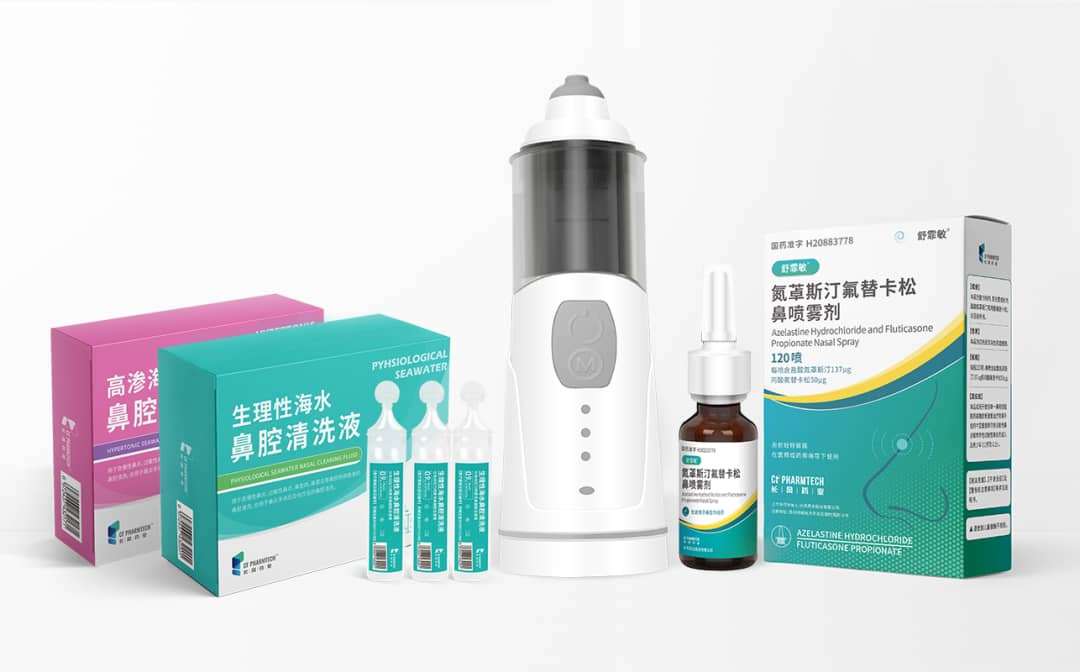
For the treatment of allergic rhinitis, CF PharmTech to Sufeimin ® As the core, combined with the first BFS sea water nasal cleaning solution with Class II medical device registration certificate in China, an integrated treatment program with drugs as the core and supplemented by nasal irrigation was constructed, providing integrated services for the treatment of patients with allergic rhinitis.
CF PharmTech always pays attention to the urgent needs of patients, focuses on the treatment of respiratory diseases, actively arranges in the fields of drugs, equipment, rehabilitation services and other fields, and provides clinicians and patients with an overall plan for the treatment of respiratory diseases throughout the course of the disease. ShuFeiMin ® The approval of azepine and fluticasone nasal spray established the core of CF PharmTech's integrated treatment plan for allergic rhinitis, comprehensively covering environmental control, drug therapy, and health education in the four-in-one treatment principle of allergic rhinitis, meeting the unmet clinical needs of patients with allergic rhinitis, and helping to improve the prevention and control level of allergic rhinitis in China. It has a milestone significance for the development of the holistic treatment of allergic rhinitis in China. Finally, look forward to ShuFeimin ® After listing, it can bring more clinical benefits to the majority of patients with allergic rhinitis.